| Infiltration and Extravasation: Update on Prevention and Management |
Darcy Doellman BSN, RN, CRNI®
Lynn Hadaway MEd, RN, BC, CRNI®
Leigh Ann Bowe-Geddes BS, RN, CRNI®
Michelle Franklin BSN, RN, MBA, CRNI®
Jack LeDonne MD
Lorelei Papke-O'Donnell MSN, RN, CRNI®
Janet Pettit MSN, RNC, NNP, CNS
Lisa Schulmeister MN, RN, APRN-BC, OCN®, FAAN
Marc Stranz PharmD
|
Journal of Infusion Nursing
July/August 2009
Volume 32 Number 4
Pages 203 - 211 |
ABSTRACT
Infiltration and extravasation are risks of intravenous administration therapy involving unintended leakage of solution into the surrounding tissue. Consequences range from local irritation to amputation. While immediate action using appropriate measures (ie, dilution, extraction, antidotes, and supportive treatments) can decrease the need for surgical intervention, many injuries may be prevented by following established policy and procedures. However, timely surgical intervention, when necessary, can prevent more serious adverse outcomes. Clinicians should be prepared to act promptly when an event occurs. Thorough incident documentation helps determine whether infusion care meets the standard of practice and is a keystone to medicolegal defense.
Infiltration-the inadvertent leakage of a nonvesicant solution into surrounding tissue-and extravasation-the inadvertent leakage of a vesicant solution into surrounding tissue1-are both known risks of intravenous (IV) therapy.2 While the injury is usually minor and resolves spontaneously,3 some cases result in serious complications, including full-thickness skin loss and muscle and tendon necrosis requiring reconstructive surgery or even amputation, leading to longer hospital stays, increased morbidity,4 and increased costs.5,6 However, management of infiltration and extravasation lacks evidence-based standardization, and many institutions do not have adequate policies and procedures in place. Furthermore, because infiltration and extravasation occur infrequently and ethical concerns prohibit controlled research, most treatments are empirical and are based on small uncontrolled trials, case reports, or animal studies.7 Additional barriers to optimal management include failure to identify the problem in a timely fashion; failure to disseminate or update management information; inadequate staffing; high staff turnover; lack of knowledge about effective treatments due to research limitations, as described above; and cost.
To better understand infiltration and extravasation, a panel of clinicians with expertise in nursing, vascular access, general surgery, and pharmacy convened in September 2007 in Phoenix, Arizona. Objectives of the advisory roundtable were to present current clinical practice, review the pertinent literature, and summarize current management options. The discussion was cochaired by Darcy Doellman, BSN, RN, CRNI(R), and Lynn Hadaway, MEd, RN, BC, CRNI(R), and made possible by an educational grant from Baxter International, Inc. While the panel discussion covered 4 key areas (prevention, recognition, management, and documentation), this article focuses primarily on the most current management options for infiltration and extravasation.
MECHANISMS OF TISSUE INJURY IN INFILTRATION AND EXTRAVASATION INJURIES
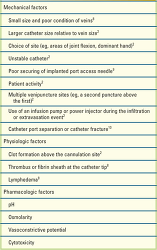
Infiltration and extravasation can be caused by mechanical, physiologic, or pharmacologic factors (Table 1). Mechanical factors, occurring either during initial catheter insertion or while the catheter is in place,2,8 and physiologic factors relating to preexisting or emerging vein problems8,11,12 can be contributing factors.9,13 Regardless of the mechanism, specific management of infiltration and extravasation is usually determined by the pharmacologic characteristics of the offending infusion.
 |
|
TABLE 1 Factors Contributing to the Risk for Infiltration and Extravasation |
While infiltration of nonvesicant agents generally does not cause tissue necrosis,8,10 the agents can sometimes result in long-term disability due to local inflammatory reactions caused by their irritant properties2,8,10,14 or by compression of surrounding tissues by a large volume of infiltrate, known as acute limb compartment syndrome (ALCS).15,16 In contrast, vesicant extravasation produces progressive tissue damage, the full extent of which may become evident only days or weeks after exposure.7,10 Variables that determine the extent of tissue damage caused by extravasation are summarized in Table 2.
 |
|
TABLE 2 Extravasant Characteristics That Determine the Extent of Injury |
Solutions that irritate the venous endothelium and vessel wall ultimately raise the risk of venous rupture, allowing the solutions to escape into the surrounding tissue.18 To minimize venous irritation, infused solutions should be close to physiologic pH (7.35-7.40)19 and osmolarity (281-282 mOsm/L).20,21 Extreme pH (both alkaline and acidic) can reduce peripheral vein tolerance22 by damaging cell proteins and eventually causing cell death, leading to venous endothelial damage and making it susceptible to rupture.21 The pH of infusion solutions should generally be between 5 and 9.21
For solutions that escape the vein, osmolarity can also influence the degree of tissue injury. For example, extravasation of hypertonic fluids such as 10% dextrose or parenteral nutrition solutions can cause skin necrosis and serious tissue damage.18,23 Furthermore, dextrose solutions are rarely administered alone: other products are often added, which can increase osmolarity even more. Extravasation of hypertonic solutions (eg, osmolarity > 350 mOsm/L) causes a fluid shift from inside cells to the interstitial space,2 which disrupts cell function.18,24 The shift from subcutaneous cells to interstitial tissues leads to swelling and increased local pressure, which can cause ALCS and other injuries.18 While hyperosmolar solutions can shrink cells and collapse the internal structures, hypoosmolar solutions can swell cells and lead to cell rupture.
Extravasation of vasoactive substances (eg, dobutamine, dopamine, epinephrine, norepinephrine, and vasopressin) can result in ischemic necrosis10,25 because these substances reduce blood flow by causing severe constriction of smooth muscles around capillaries.4,17 In addition, solutions with high electrolyte concentrations (eg, calcium chloride 5.5% or sodium chloride 3% or 5%) can prolong the depolarization and contraction of pre- and postcapillary smooth muscle sphincters, which, in turn, prolongs exposure to injurious substances and leads to ischemia and tissue necrosis.4,10,17
Many antineoplastic agents can cause direct cellular toxicity upon extravasation.10 Such agents can be separated into 2 categories on the basis of their mechanisms of cellular damage: nonbinding vesicants (agents that do not bind to tissue nucleic acids) and DNA-binding vesicants (agents that bind to tissue nucleic acids).11 The most destructive extravasation injuries are caused by the DNA-binding agents (eg, anthracyclines, antitumor antibiotics, and some alkylating agents),8 which cause immediate tissue injury and-by remaining in the tissues-create a more prolonged course.11 In contrast, nonbinding vesicant agents (eg, vinca alkaloids and taxanes)8 cause immediate tissue damage, but because they do not bind to DNA, these drugs are more easily metabolized, and tissue repair follows a more normal healing process.11 Even agents that are not antineoplastics-such as antibiotics-can cause depletion of intracellular ADP and ATP levels and other enzymes that sustain cell function, leading to cell death. The impact appears to depend on drug concentration and time of exposure.
MANAGEMENT
While it is widely recognized that early identification and intervention upon the first signs and symptoms of infiltration and extravasation are critical to the prevention of potentially serious adverse outcomes, definitive treatment has not been established, with the exception of dexrazoxane hydrochloride (Totect(R), TopoTarget USA, Rockaway, New Jersey), which is a Food and Drug Administration (FDA)-approved anthracycline extravasation treatment.26 Consequently, current management recommendations are based for the most part on anecdotal experience.2,27-29 However, all current guidelines recommend the following steps at the first sign of infiltration or extravasation: (1) stop administration of IV fluids immediately; (2) disconnect the IV tubing from the device; (3) attempt aspiration of the residual drug from the IV device; (4) administer nursing interventions (summarized below), as indicated; and (5) notify the physician or advanced practice nurse.29-31
Beginning with the most conservative approach, the following sections describe the currently available treatment options: supportive care; manual extraction of the extravasated fluid; use of dispersal agents, antidotes, and treatments; and surgical excision of the extravasation site.27,28 An extravasation kit containing the items needed to assess the IV site and to treat an extravasation injury should be readily accessible. Useful kit items include the institution's extravasation policy, management algorithm, and documentation form; 3-mL syringes; 25-gauge needles; cold and warm compresses; and paper tape.28 Some institutions' kits also include specific antidotes, with appropriate diluent and reconstitution instructions, and a tape measure to determine the size of the involved area.
Nursing interventions include elevation and thermal application (cold or heat). Elevation of the affected limb may aid in reabsorption of the infiltrate or extravasated vesicant by decreasing capillary hydrostatic pressure.3,31,32 Although one study of limb elevation (of approximately 2-4 in) did not demonstrate alleviation of pain or resolution of infiltrate,33 elevation of the affected limb is recommended for 24 to 48 hours after infiltration or extravasation, whenever possible.29-32
Local thermal treatments are used to decrease the site reaction and absorption of the infiltrate.28 Local cooling (ice packs) aids in vasoconstriction, thus theoretically limiting drug dispersion.31 Cold application is recommended for extravasation of DNA-binding vesicants (with the exception of mechlorethamine [nitrogen mustard]),30 contrast media,3,34 and hyperosmolar fluids.2,35 The use of local warming therapy (dry heat) is based on the theory that it enhances vasodilation, thus enhancing dispersion of the vesicant agent and decreasing drug accumulation in the local tissue.31 The use of local warming is recommended for extravasation of non-DNA-binding vesicants.30 Although clear benefit has not been demonstrated with thermal applications,17,35,36 it remains standard supportive care, and the recommended application schedule for both warm and cold applications is 15 to 20 minutes, every 4 hours, for 24 to 48 hours.30 It should be noted that heat and cold applications are not well supported in neonates and young infants.
As soon as infiltration or extravasation has been identified, the infusion should be stopped, the IV tubing should be disconnected (leaving the catheter in place), and then an attempt should be made to aspirate the residual drug from the IV device using a small (1-to 3-mL) syringe.30
Documented experience with methods of manually extracting infiltrated or extravasated agents is lacking, so these techniques are not routinely recommended, and should be performed only by a physician or other qualified personnel. The "squeeze maneuver" was described in a report of 8 patients with more than 50 mL of contrast-media extravasation that had resulted in vascular compromise of the fingers. After stopping the infusion, the IV catheter was removed and an 18-gauge needle was used to create 5 to 8 holes near the insertion site (avoiding vessels, tendons, and muscles). The patients' tissue was then "milked" from the edges of the distended area toward the needle holes, squeezing out the extravasated contrast media until distal circulation had been restored.37 The advantages of this procedure are that it can be performed immediately and it does not require anesthesia37; however, experience with its use is limited.
Other reported manual extraction methods include percutaneous needle aspiration, liposuction, and surgical fenestration and irrigation.27 Early washout using surgical fenestration and irrigation has been described as a simple, practical procedure,23,38 which immediately reduces the amount of extravasated agent at the site with good outcomes. In an early report, 44 pediatric patients with extravasations of calcium, potassium, sodium bicarbonate, or 10% dextrose were treated within 24 hours of extravasation injury with stab incisions and 500 mL of normal saline flush, with drains left in place for 24 hours. In these patients, 86% healed without soft tissue loss.23 In a later report, 8 patients with suspected vinca alkaloid or anthracycline chemotherapy extravasations were treated with several incisions within 6 hours of the extravasation, followed by infiltration with 300 to 500 mL of normal saline via a large catheter.38 All 8 patients experienced normal healing without functional impairment. Although these early reports of saline washout were positive, they have limited clinical utility and have not been incorporated into infusion guidelines.30
The theory behind the use of dispersal is that tissue injury will be decreased secondary to the dilution of the vesicant across a larger area of tissue.29 Clysis with saline has been described as the simplest method to dilute the concentration of a vesicant extravasation in order to prevent tissue injury.27 In a 1994 report on 40 patients with suspected extravasations of vinca alkaloids or doxorubicin, conservative treatment with 20 to 90 mL of saline solution, injected at the extravasation site 3 to 6 times, was administered over a course of several days to all patients except 3 with deep lesions.39 Pain and erythema were resolved in 4 days or less and superficial ulcerations in 10 to 14 days in all treated patients; surgery was required in the 3 patients with deep ulcers. Variations of the saline wash procedure include the saline flush-out technique described in the preceding section.
Although a number of pharmacologic antidotes have been investigated for management of vesicant extravasation,12,40 their use remains controversial.2 While several antidotes described in this section have been shown to limit tissue damage caused by extravasation of specific vesicants and are recommended in empirical guidelines,8,28,29,32,41 the most recent Oncology Nursing Society guidelines do not recommend antidotes for extravasation of chemotherapeutic and biotherapeutic agents, with the exception of sodium thiosulfate.30 Therefore, the package insert or other reference material should be consulted before considering the use of an antidote for management of extravasation of specific agents.7
Hyaluronidase enzymatically increases tissue permeability, which facilitates systemic absorption of infiltrated vesicant agents.29 Hyaluronidase rapidly (within 10 minutes) results in diffusion of extravasated fluid over an area 3 to 5 times larger than an area left untreated, and tissue permeability is restored within 24 to 48 hours.42
In both animal studies and human reports, good outcomes have been observed with hyaluronidase treatment of suspected extravasations of DNA-binding drugs (eg, doxorubicin),43,44 non-DNA-binding drugs (eg, vinca alkaloids),45,46 irritant drugs (eg, nafcillin),42 hyperosmolar solutions (eg, dextrose 10%, parenteral nutrition),42 and many other agents. In a recent case report on parenteral nutrition extravasation in a premature infant, early subcutaneous hyaluronidase followed by saline flushing resulted in a dramatic response with almost no sign of injury after 5 days.47 Hyaluronidase is not recommended for use with dopamine or [alpha]-agonist drugs.48 Clinicians should consider patients with allergies and with religious/cultural proscriptions when choosing between animal-derived forms of hyaluronidase and the new recombinant human form (rHuPH20).48
Sodium thiosulfate has long been recognized as an effective antidote to mechlorethamine (nitrogen mustard).40 Furthermore, a study of 63 patients who had injuries induced by extravasation of a variety of vesicant agents (eg, doxorubicin, epirubicin, vinblastine, and mitomycin C) showed that sodium thiosulfate combined with conservative treatment significantly improved healing time compared with conservative treatment alone.49 The recommended administration of sodium thiosulfate is through immediate subcutaneous injection of 2 mL of 0.17M solution.50
Dimethyl sulfoxide (DMSO) is a topically applied solvent that may improve systemic absorption of extravasated vesicants. It also acts as a free radical scavenger, thus preventing DNA damage from oxygen free radicals that might be produced by cytotoxic agents.40,41 Topical administration of DMSO after vesicant drug extravasation has been shown to prevent tissue necrosis in several animal studies51,52; however, in another study, DMSO failed to reduce ulceration after anthracycline extravasation53; and a third study suggested that DMSO might potentiate, rather than inhibit, the toxicity of cytotoxic agents.54 Although good results have been reported in clinical trials in patients with suspected extravasations of cytotoxic agents using 90% to 99% DMSO,55-57 medical-grade DMSO at concentrations higher than 50% is not available in the United States.31 Because of conflicting efficacy data, limited drug availability, and the advent of new treatments such as Totect(R), the use of DMSO as an antidote is not recommended in the Oncology Nursing Society guidelines.30
Extravasation of vasopressor agents (eg, dopamine, epinephrine, and norepinephrine) causes local vasoconstriction and ischemia, which can result in tissue necrosis and ulceration.58 Phentolamine competitively blocks [alpha]-adrenergic receptors, reversing these effects, thereby mitigating the tissue injury. As early as 1957, phentolamine was reported to reduce skin loss caused by extravasation of vasoconstrictor agents in animal models and in case studies.59 While one early report described a vasopressor extravasation injury that did not respond to phentolamine administered 48 hours after extravasation,60 a later animal study demonstrated that early administration of phentolamine was critical to its beneficial effects on vasopressor extravasation injuries.61 Results of these animal studies are supported by the findings of several case reports,62,63 and administration of phentolamine no more than 12 hours after extravasation is supported in empirical guidelines for the management of vasopressor extravasation in both children32,58 and adults.17
Totect(R) is a new FDA-approved treatment for IV anthracycline extravasation.64 The mechanism by which it reduces tissue damage is unknown.64 Efficacy in treating anthracycline extravasations has been demonstrated in animal studies,65-67 case reports,68-71 and 2 multicenter, prospective clinical trials.72 In the 2 clinical trials, 54 patients with biopsy-confirmed doxorubicin and epirubicin extravasations received 3 days of IV dexrazoxane (1000, 1000, and 500 mg/m2) treatment beginning no later than 6 hours after the event.72 Skin and tissue integrity remained intact in 53 of 54 patients (98.2% efficacy); only 1 of the 54 patients (2.8%) required surgical resection of necrosis. Sequelae were reported as mild among those not requiring surgery, and 74% of the patients were able to continue anthracycline chemotherapy on schedule.
Totect(R) is packaged as an emergency treatment kit for single patient use and contains a complete course of treatment. The drug is administered for 3 consecutive days as a 1-to 2-hour IV infusion in a large-caliber vein in an extremity other than the one affected by extravasation. The recommended dose is the same as that administered in the clinical trials64: 1000 mg/m2 on day 1; 1000 mg/m2 on day 2, and 500 mg/m2 on day 3.
The majority of suspected extravasation injuries are believed to heal without surgical intervention, so a conservative approach is advisable.3,27,29,73,74 However, it has been estimated that surgery is required for up to one-third of cases.29,40,75 Therefore, timely surgical consultation is important to minimize adverse outcomes when extravasation of nonanthracycline vesicants occurs (anthracycline extravasations are immediately treated with Totect(R)).2,28,39 Continuing pain after administration of conservative local treatment has been cited as an indication for surgical consultation.11,29,40,76 When surgery is indicated, early debridement of necrotic tissues and entrapped drug can minimize the risk for subsequent damage to deeper tissues; and when healing is delayed, wide excision and skin grafting are the most common procedures.40,76 More aggressive surgery, such as mastectomy, may be required in cases of extensive central venous catheter extravasation injury.9
In contrast, the primary treatment of ALCS is decompression with fasciotomy.16 Because outcomes are optimal when decompression occurs less than 12 hours after the onset of ALCS,77,78 surgical treatment should be initiated as soon as possible to minimize complications.16
Hyperbaric oxygen therapy involves intermittent inhalation of 100% oxygen in a treatment chamber with pressure greater than 1 atm.79,80 The rationale is that intermittently increasing tissue oxygen tension optimizes fibroblast proliferation and collagen synthesis, thereby enhancing the oxidative killing capacity of white blood cells during hyperoxia and stimulating angiogenesis during hypoxia.80 Enhancement of healing in selected problem wounds is one of the 13 indications approved for hyperbaric oxygen therapy by the Hyperbaric Oxygen Committee of the Underwater and Hyperbaric Medical Society.81
Studies of hyperbaric oxygen therapy in animal models of doxorubicin extravasation have reported mixed results. In an early study, ulcers in rats treated with hyperbaric oxygen therapy were significantly larger at 2, 3, and 5 weeks as compared with those in untreated animals, suggesting that cytotoxic effects of doxorubicin were potentiated by this treatment modality.80 In contrast, a more recent study using a similar model reported no difference in lesion size in hyperbaric-oxygen-treated animals and in control animals on days 7 or 14, but a significant reduction in the treated animals on days 21 and 28, with complete wound healing by day 40 in 37% of the treated animals versus no wound healing in the control group.79 These findings are preliminary, and currently there are no reports of hyperbaric oxygen therapy in humans for the treatment of tissue necrosis caused by vesicant extravasation. Moreover, the use of hyperbaric oxygen therapy is not discussed in the current guidelines for the practice of chemotherapy and biotherapy.30
The theoretical mechanism of hyperbaric oxygen therapy for the treatment of ALCS is that hyperoxygenation increases the supply of oxygen and causes vasoconstriction, resulting in decreased capillary blood pressure and transudation.16 Hyperbaric oxygen therapy has been shown to reduce edema and tissue necrosis in experimental models of ALCS82,83 and promote wound healing in patients with ALCS resulting from traumatic injury. In one study, 17 of 18 patients who were given hyperbaric treatment following surgical fasciotomy experienced complete wound healing as compared with 10 of 18 patients who received fasciotomy alone.84 Hyperbaric therapy is currently recommended as an adjunct to fasciotomy or in those cases in which immediate surgical treatment is not possible.16
Total parenteral nutrition, electrolyte therapy, antibiotics, vasoactive medications, and other drug therapies requiring peripheral IV therapy are often needed in low-birth-weight infants and in children with complex illnesses.58 However, their small, fragile veins and inability to verbally communicate their pain and discomfort leave them particularly prone to infiltration and extravasation injuries.29,85 Although wound and skin care is emphasized in newborns and infants,18 treatment for children, like that for adults, is based on the anecdotal literature.32 The value of hot or cold compresses is debatable; there is little evidence of efficacy and some risk of skin maceration with moist heat.
IMPORTANCE OF PREVENTION AND RECOGNITION
In view of the current controversy regarding the indications for some treatments and the limitations of others, prevention has been correctly stated to be preferable to cure.86 Careful adherence to proper procedures and timely identification of signs and symptoms are critical to avoiding potentially life-altering complications.2,28 Although the primary responsibility for the prevention and early recognition of injury due to infiltration and extravasation rests with the nursing staff,2,8 physicians and pharmacists should be knowledgeable about the most up-to-date treatment options and institutional protocols and should be prepared to act promptly in order to prevent serious complications.
|
DOCUMENTATION
Because errors associated with IV administration can result in fatal or life-threatening outcomes, administration of IV fluids and medications can be high-risk, with adverse outcomes potentially leading to malpractice claims.5 While nurses are named as defendants in such lawsuits in increasing numbers, physicians also can be named if it appears that intervention was not proper or timely. For example, the Closed Claims Project database, which summarizes data from professional liability carriers, showed that 2.1% of injury claims from 1970 to 2001 were related to peripheral catheters.6 Among these claims, 28% were related to skin slough or necrosis; 17% were related to swelling, inflammation, or infection; 17% were related to nerve damage (with 22% of these caused by ALCS); 16% were related to fasciotomy scars resulting from ALCS; and 3% were related to heat compresses used to treat IV infiltrations.6 Approximately 54% of peripheral catheter claims resulted in successful litigation for the plaintiffs, with compensation ranging from $275 to $10,050,000.6
Evidence-based management and complete and accurate documentation are the keys to an effective legal defense in the event of a medicolegal claim.5 The Joint Commission defines a sentinel event as "an unexpected occurrence involving death or serious physical or psychological injury, or the risk thereof. Serious injury specifically includes loss of limb or function."87 According to the Infusion Nursing Standards of Practice, an extravasation injury should be considered a sentinel event and should be documented.1 Extreme cases of infiltration and all extravasations require a sentinel event report, which then triggers a root cause analysis. Risk managers should encourage complete and accurate documentation of all infiltration and extravasation events and should analyze such events for system failure and identify opportunities for process improvement. The most important documentation component is a comprehensive, standardized form to ensure that complete detailed information is gathered (Table 3). A photograph of the affected site should also be considered an important component of the documentation record.8
 |
|
TABLE 3 Content of Standardized Event Documentation Form |
SUMMARY
Infiltration and extravasation injuries are medical emergencies that have the potential to cause serious disability, diminish the patient's quality of life, and leave clinicians vulnerable to the risk of malpractice claims. While such injuries may be minimized or prevented through adherence to standards of practice and evidence-based treatment, further study is needed to address unresolved questions and controversies surrounding extravasation management and ways to broaden clinicians' awareness of the treatment options.
REFERENCES
1. Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2006;29(1)(suppl):S1-S92. [Context Link]
2. Hadaway L. Infiltration and extravasation: preventing a complication of IV catheterization. Am J Nurs. 2007;107(8):64-72. [Context Link]
3. Bellin MF, Jakobsen JA, Tomassin I, et al. Contrast medium extravasation injury: guidelines for prevention and management. Eur Radiol. 2002;12(11):2807-2812. [Context Link]
4. Upton J, Mulliken JB, Murray JE. Major intravenous extravasation injuries. Am J Surg. 1979;137(4):497-506. [Context Link]
5. Diehl-Svrjcek BC, Dawson B, Duncan LL. Infusion nursing: aspects of practice liability. J Infus Nurs. 2007;30(5):274-279. [Context Link]
6. Liau DW. Injuries and liability related to peripheral catheters: a Closed Claims analysis. Newsl Am Soc Anesthesiol. 2006;70(6). http://www.asahq.org/Newsletters/2006/06-06/liau06_06.html. Accessed June 4, 2008. [Context Link]
7. Ener RA, Meglathery SB, Styler M. Extravasation of systemic hemato-oncological therapies. Ann Oncol. 2004;15(6):858-862. [Context Link]
8. Goolsby TV, Lombardo FA. Extravasation of chemotherapeutic agents: prevention and treatment. Semin Oncol. 2006;33(1):139-143. [Context Link]
9. Schulmeister L, Camp-Sorrell D. Chemotherapy extravasation from implanted ports. Oncol Nurs Forum. 2000;27(3):531-538. [Context Link]
10. Sauerland C, Engelking C, Wickham R, Corbi D. Vesicant extravasation, I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33(6):1134-1141. [Context Link]
11. Rudolph R, Larson DL. Etiology and treatment of chemotherapeutic agent extravasation injuries: a review. J Clin Oncol. 1987;5(7):1116-1126. [Context Link]
12. Ignoffo RJ, Friedman MA. Therapy of local toxicities caused by extravasation of cancer chemotherapeutic drugs. Cancer Treat Rev. 1980;7(1):17-27. [Context Link]
13. Haynes S. Infusion phlebitis and extravasation. Prof Nurse. 1989;5(3):160-161. [Context Link]
14. Luke E. Mitoxantrone-induced extravasation. Oncol Nurs Forum. 2005;32(1):27-29. [Context Link]
15. Mubarak SJ, Hargens AR. Acute compartment syndromes. Surg Clin North Am. 1983;63(3):539-565. [Context Link]
16. Tiwari A, Haq AI, Myint F, Hamilton G. Acute compartment syndromes. Br J Surg. 2002;89(4):397-412. [Context Link]
17. Schummer W, Schummer C, Bayer O, Muller A, Bredle D, Karzai W. Extravasation injury in the perioperative setting. Anesth Analg. 2005;100(3):722-727. [Context Link]
18. Pettit J, Hughes K. Intravenous extravasation: mechanisms, management, and prevention. J Perinat Neonatal Nurs. 1993;6(4):69-78. [Context Link]
19. Guyton AC, Hall JE. Regulation of acid-base balance. In: Guyton AC, Hall JE, eds. Textbook of Medical Physiology. 10th ed. Philadelphia, PA: WB Saunders; 2000:346-363. [Context Link]
20. Guyton AC, Hall JE. The body fluid compartments: extracellular and intracellular fluids: interstitial fluid and edema. In: Guyton AC, Hall JE, eds. Textbook of Medical Physiology. 10th ed. Philadelphia, PA: WB Saunders; 2000:264-278. [Context Link]
21. Vesely TM, Stranz M, Masoorli S, Hadaway LC. The diverse and conflicting standards and practices in infusion therapy. J Vasc Access Devices. 2002;7(3):9-25. [Context Link]
22. Kuwahara T, Asanami S, Kubo S. Experimental infusion phlebitis: tolerance osmolality of peripheral venous endothelial cells. Nutrition. 1998;14(6):496-501. [Context Link]
23. Gault DT. Extravasation injuries. Br J Plast Surg. 1993;46(2):91-96. [Context Link]
24. Robijns BJ, de Wit WM, Bosma NJ, van Vloten WA. Localized bullous eruptions caused by extravasation of commonly used intravenous infusion fluids. Dermatologica. 1991;182(1):39-42. [Context Link]
25. Gaze NR. Tissue necrosis caused by commonly used intravenous infusions. Lancet. 1978;2(8086):417-419. [Context Link]
26. Totect(R) [package insert]. Rockaway, NJ: TopoTarget USA Inc; 2007. [Context Link]
27. Langstein HN, Duman H, Seelig D, Butler CE, Evans GR. Retrospective study of the management of chemotherapeutic extravasation injury. Ann Plast Surg. 2002;49(4):369-374. [Context Link]
28. Wickham R, Engelking C, Sauerland C, Corbi D. Vesicant extravasation, II: evidence-based management and continuing controversies. Oncol Nurs Forum. 2006;33(6):1143-1150. [Context Link]
29. Kassner E. Evaluation and treatment of chemotherapy extravasation injuries. J Pediatr Oncol Nurs. 2000;17(3):135-148. [Context Link]
30. Polovich M, White JM, Kelleher LO. Immediate complications of cytotoxic therapy. In: Polovich M, White JM, Kelleher LO, eds. Chemotherapy and Biotherapy Guidelines and Recommendations for Practice. Pittsburgh, PA: Oncology Nursing Society; 2005:78-88. [Context Link]
31. Schulmeister L. Extravasation management. Semin Oncol Nurs. 2007;23(3):184-190. [Context Link]
32. Montgomery LA, Hanrahan K, Kottman K, Otto A, Barrett T, Hermiston B. Guideline for IV infiltrations in pediatric patients. Pediatr Nurs. 1999;25(2):167-180. [Context Link]
33. Yucha CB, Hastings-Tolsma M, Szeverenyi NM. Effect of elevation on intravenous extravasations. J Intraven Nurs. 1994;17(5):231-234. [Context Link]
34. Elam EA, Dorr RT, Lagel KE, Pond GD. Cutaneous ulceration due to contrast extravasation: experimental assessment of injury and potential antidotes. Invest Radiol. 1991;26(1):13-16. [Context Link]
35. Hastings-Tolsma MT, Yucha CB, Tompkins J, Robson L, Szeverenyi N. Effect of warm and cold applications on the resolution of IV infiltrations. Res Nurs Health. 1993;16(3):171-178. [Context Link]
36. Hastings-Tolsma M, Yucha CB. IV infiltration no clear signs, no clear treatment? RN. 1994;57(12):34-38. [Context Link]
37. Tsai YS, Cheng SM, Ng SP, et al. Squeeze maneuver: an easy way to manage radiological contrast-medium extravasation. Acta Radiol. 2007;48(6):605-607. [Context Link]
38. Giunta R. Early subcutaneous wash-out in acute extravasations. Ann Oncol. 2004;15(7):1146. [Context Link]
39. Scuderi N, Onesti MG. Antitumor agents: extravasation, management, and surgical treatment. Ann Plast Surg. 1994;32(1):39-44. [Context Link]
40. Dorr RT. Antidotes to vesicant chemotherapy extravasations. Blood Rev. 1990;4(1):41-60. [Context Link]
41. Bertelli G. Prevention and management of extravasation of cytotoxic drugs. Drug Saf. 1995;12(4):245-255. [Context Link]
42. Zenk KE, Dungy CI, Greene GR. Nafcillin extravasation injury: use of hyaluronidase as an antidote. Am J Dis Child. 1981;135(12):1113-1114. [Context Link]
43. Disa JJ, Chang RR, Mucci SJ, Goldberg NH. Prevention of adriamycin-induced full-thickness skin loss using hyaluronidase infiltration. Plast Reconstr Surg. 1998;101(2):370-374. [Context Link]
44. Heckler FR. Current thoughts on extravasation injuries. Clin Plast Surg. 1989;16(3):557-563. [Context Link]
45. Dorr RT, Alberts DS. Vinca alkaloid skin toxicity: antidote and drug disposition studies in the mouse. J Natl Cancer Inst. 1985;74(1):113-120. [Context Link]
46. Bertelli G, Dini D, Forno GB, et al. Hyaluronidase as an antidote to extravasation of Vinca alkaloids: clinical results. J Cancer Res Clin Oncol. 1994;120(8):505-506. [Context Link]
47. Siu SLY, Kwong KL, Poon SST, So KT. The use of hyaluronidase for treatment of extravasations in a premature infant. Hong Kong J Paediatr. 2007;12:130-132. [Context Link]
48. Hylenex(R) [package insert]. Deerfield, IL: Baxter Healthcare Corporation; 2008. [Context Link]
49. Tsavaris NB, Komitsopoulou P, Karagiaouris P, et al. Prevention of tissue necrosis due to accidental extravasation of cytostatic drugs by a conservative approach. Cancer Chemother Pharmacol. 1992;30(4):330-333. [Context Link]
50. Schrijvers DL. Extravasation: a dreaded complication of chemotherapy. Ann Oncol. 2003;14(suppl 3):iii26-iii30. [Context Link]
51. Dorr RT, Soble MJ, Liddil JD, Keller JH. Mitomycin C skin toxicity studies in mice: reduced ulceration and altered pharmacokinetics with topical dimethyl sulfoxide. J Clin Oncol. 1986;4(9):1399-1404. [Context Link]
52. Lebredo L, Barrie R, Woltering EA. DMSO protects against adriamycin-induced tissue necrosis. J Surg Res. 1992;53(1):62-65. [Context Link]
53. Dorr RT, Alberts DS. Failure of DMSO and vitamin E to prevent doxorubicin skin ulceration in the mouse. Cancer Treat Rep. 1983;67(5):499-501. [Context Link]
54. Pommier RF, Woltering EA, Fletcher WS. Synergistic cytotoxicity of DMSO-antineoplastic combinations against P388 leukemia in CD-F1 mice. Surg Forum. 1988;39:471-473. [Context Link]
55. Ludwig CU, Stoll HR, Obrist R, Obrecht JP. Prevention of cytotoxic drug induced skin ulcers with dimethyl sulfoxide (DMSO) and alpha-tocopherole. Eur J Cancer Clin Oncol. 1987;23(3):327-329. [Context Link]
56. Bertelli G, Gozza A, Forno GB, et al. Topical dimethyl sulfoxide for the prevention of soft tissue injury after extravasation of vesicant cytotoxic drugs: a prospective clinical study. J Clin Oncol. 1995;13(11):2851-2855. [Context Link]
57. Olver IN, Aisner J, Hament A, Buchanan L, Bishop JF, Kaplan RS. A prospective study of topical dimethyl sulfoxide for treating anthracycline extravasation. J Clin Oncol. 1988;6(11):1732-1735. [Context Link]
58. Flemmer L, Chan JS. A pediatric protocol for management of extravasation injuries. Pediatr Nurs. 1993;19(4):355-358, 424. [Context Link]
59. Zucker G. Use of phentolamine to prevent necrosis due to levarterenol. JAMA. 1957;163(16):1477-1479. [Context Link]
60. Boltax RS, Dineen JP, Scarpa FJ. Gangrene resulting from infiltrated dopamine solution. N Engl J Med. 1977;296(14):823. [Context Link]
61. Aycock BG, Hawtof DB, Moody SB. Treatment of peripheral ischemia secondary to lidocaine containing epinephrine. Ann Plast Surg. 1989;23(1):27-30. [Context Link]
62. Alexander CS, Sako Y, Mikulic E. Pedal gangrene associated with the use of dopamine [medical intelligence]. N Engl J Med. 1975;293(12):591. [Context Link]
63. Greenlaw CW, Null LW. Dopamine-induced ischaemia [letter]. Lancet. 1977;2(8037):555. [Context Link]
64. Siwy BK, Sadove AM. Acute management of dopamine infiltration injury with Regitine. Plast Reconstr Surg. 1987;80(4):610-612. [Context Link]
65. Langer SW, Sehested M, Jensen PB. Treatment of anthracycline extravasation with dexrazoxane. Clin Cancer Res. 2000;6(9):3680-3686. [Context Link]
66. Langer SW, Sehested M, Jensen PB. Dexrazoxane is a potent and specific inhibitor of anthracycline induced subcutaneous lesions in mice. Ann Oncol. 2001;12(3):405-410. [Context Link]
67. Langer SW, Thougaard AV, Sehested M, Jensen PB. Treatment of anthracycline extravasation in mice with dexrazoxane with or without DMSO and hydrocortisone. Cancer Chemother Pharmacol. 2006;57(1):125-128. [Context Link]
68. Bos AM, van der Graaf WT, Willemse PH. A new conservative approach to extravasation of anthracyclines with dimethylsulfoxide and dexrazoxane. Acta Oncol. 2001;40(4):541-542. [Context Link]
69. El Saghir N, Otrock Z, Mufarrij A, et al. Dexrazoxane for anthracycline extravasation and GM-CSF for skin ulceration and wound healing. Lancet Oncol. 2004;5(5):320-321. [Context Link]
70. Jensen JN, Lock-Andersen J, Langer SW, Mejer J. Dexrazoxane-a promising antidote in the treatment of accidental extravasation of anthracyclines. Scand J Plast Reconstr Surg Hand Surg. 2003;37(3):174-175. [Context Link]
71. Langer SW, Sehested M, Jensen PB, Buter J, Giaccone G. Dexrazoxane in anthracycline extravasation [correspondence]. J Clin Oncol. 2000;18(16):3064. [Context Link]
72. Mouridsen HT, Langer SW, Buter J, et al. Treatment of anthracycline extravasation with Savene (dexrazoxane): results from two prospective clinical multicentre studies. Ann Oncol. 2007;18(3):546-550. [Context Link]
73. Bozkurt AK, Uzel B, Akman C, Ozguroglu M, Molinas MN. Intrathoracic extravasation of antineoplastic agents: case report and systematic review. Am J Clin Oncol. 2003;26(2):121-123. [Context Link]
74. Cohan RH, Dunnick NR, Leder RA, Baker ME. Extravasation of nonionic radiologic contrast media: efficacy of conservative treatment. Radiology. 1990;176(1):65-67. [Context Link]
75. Larson DL. Treatment of tissue extravasation by antitumor agents. Cancer. 1982;49(9):1796-1799. [Context Link]
76. Boyle DM, Engelking C. Vesicant extravasation: myths and realities. Oncol Nurs Forum. 1995;22(1):57-67. [Context Link]
77. Sheridan GW, Matsen FA III. Fasciotomy in the treatment of the acute compartment syndrome. J Bone Joint Surg Am. 1976;58(1):112-115. [Context Link]
78. Mullett H, Al-Abed K, Prasad CV, O'Sullivan M. Outcome of compartment syndrome following intramedullary nailing of tibial diaphyseal fractures. Injury. 2001;32(5):411-413. [Context Link]
79. Aktas S, Toklu AS, Olgac V. Hyperbaric oxygen therapy in adriamycin extravasation: an experimental animal study. Ann Plast Surg. 2000;45(2):167-171. [Context Link]
80. Monstrey SJ, Mullick P, Narayanan K, Ramasastry SS. Hyperbaric oxygen therapy and free radical production: an experimental study in doxorubicin (Adriamycin) extravasation injuries. Ann Plast Surg. 1997;38(2):163-168. [Context Link]
81. Undersea & Hyperbaric Medical Society. Indications for hyperbaric oxygen therapy. http://www.uhms.org/ResourceLibrary/Indications/tabid/270/Default.aspx. Accessed June 4, 2008. [Context Link]
82. Strauss MB, Hargens AR, Gershuni DH, et al. Reduction of skeletal muscle necrosis using intermittent hyperbaric oxygen in a model compartment syndrome. J Bone Joint Surg Am. 1983;65(5):656-662. [Context Link]
83. Skyhar MJ, Hargens AR, Strauss MB, Gershuni DH, Hart GB, Akeson WH. Hyperbaric oxygen reduces edema and necrosis of skeletal muscle in compartment syndromes associated with hemorrhagic hypotension. J Bone Joint Surg Am. 1986;68(8):1218-1224. [Context Link]
84. Bouachour G, Cronier P, Gouello JP, Toulemonde JL, Talha A, Alquier P. Hyperbaric oxygen therapy in the management of crush injuries: a randomized double-blind placebo-controlled clinical trial. J Trauma. 1996;41(2):333-339. [Context Link]
85. Phelps SJ, Helms RA. Risk factors affecting infiltration of peripheral venous lines in infants. J Pediatr. 1987;111(3):384-389. [Context Link]
86. Burd DAR, Santis G, Milward TM. Severe extravasation injury: an avoidable iatrogenic disaster? BMJ. 1985;290:1579-1580. [Context Link]
87. The Joint Commission. Sentinel event. http://www.jointcommission.org/SentinelEvents/. Accessed June 4, 2008. [Context Link]
|